Iron Oxide Reacts With Aluminium
In this demonstration, students find the highly exothermic reaction betwixt aluminium and iron(Three) oxide that produces molten fe. This competition reaction proves that aluminium is a more than reactive metal than fe, while the experiment as well provides an example of a redox reaction taking place
The reaction is violent merely prophylactic provided the procedures are followed exactly. Some teachers have had accidents when performing the procedure outside in a strong breeze; the powders blew into the flame, defenseless burn and acquired burns to the hand and/or face. Siting the demonstration in a fume closet has caused damage to the cupboard.
The method described here is performed on a laboratory bench and produces express fumes. Exercise NOT do this sit-in in a fume closet or outdoors. It produces a result within seconds of setting it off considering the water cools the iron down very apace. A rehearsal is essential if this experiment has not been done before.
At that place have been occasional reported explosions when using methods similar to this. It is essential non to exceed the stated quantities and that the demonstrator and students are protected by safety screens.
The bench should be clear of combustible materials and protected with a canvass of hardboard or rut-resistant mats. The demonstrator must have room to move quickly away to a safe distance.
The demonstration takes almost 10 minutes to carry out if the appliance is set up and the solid reagents are weighed in advance.
Equipment
Apparatus
- Heart protection (note 2)
- Safety screens (note 3)
- Filter paper, 11 cm bore
- Pipeclay triangle
- Tripod
- Plastic beaker or thick-walled glass chalice, 1 dm3 (must fit between tripod legs)
- Sand (see diagram)
- Rut-resistant mats
- Plastic magnetic retriever or modest bar magnet
Chemicals
For the thermite mixture (note 6):
- Aluminium powder (medium course) (HIGHLY Flammable), three thou
- Iron(3) oxide, 9 thou
For the igniter (notes 9 and ten):
- Domestic sparkler, sixteen cm long (sold for indoor use)
Health, prophylactic and technical notes
- Read our standard health and safe guidance.
- Both the demonstrator and all observers must vesture middle protection. For the demonstrator this must be goggles or a confront shield.
- Safety screens must be used to surround the apparatus. In add-on to wearing eye protection, students should stand further than 4 1000 from the reaction.
- The demonstrator should wear a laboratory coat (the experiment can become messy at the end).
- The thermite reaction tin can trigger heat or fume sensors; yous cannot do the experiment in a laboratory fitted with fume sensors.
- It is important that the iron(III) oxide used in this sit-in is absolutely dry. An hour or so in a warm oven, or heating in an evaporating dish over a Bunsen flame, should suffice. The oxide should exist allowed to cool completely before mixing. The weighed quantities of iron(Iii) oxide (nine thou maximum) and aluminium pulverization (3 g maximum) may exist thoroughly mixed beforehand by repeatedly pouring the mixture to and fro between two pieces of scrap paper (never stir with a metal spatula), and and so stored for the demonstration in a suitable container labelled 'Thermite mixture'.
- Aluminium powder, Al(south), (HIGHLY FLAMMABLE) – see CLEAPSS Hazcard HC001A.
- Iron(III) oxide, Iron2O3(southward) – come across CLEAPSS Hazcard HC055A.
- The Igniter for the reaction is a domestic sparkler. It should be one for indoor use but need not be handheld. The sparkler needs to exist longer than 10 cm to ensure there is time to retreat to a safe altitude. A 16 cm sparkler length is ideal and the handle should be cut off using tin snips or pliers. Sparklers longer than sixteen cm may become top-heavy. Avoid cutting into backlog explosive; this causes it to cleft, and it tin can autumn off dangerously before the demonstrator tin can retire to a prophylactic distance.
- Due to the Britain Explosives Regulations 2014 alternative igniter mixtures (such as magnesium pulverization, barium nitrate and magnesium ribbon) on scales larger than 0.five g combined weight cannot be used in schools without an Explosives Certificate issued by the police force. Igniter mixtures at or below 0.5 grand issue in a less reliable ignition, so a sparkler should be used.
The demonstrator may wish (or be persuaded past the audience) to practise a repeat demonstration. In this result information technology is of import to keep the 2d gear up of materials well away from the beginning demonstration site.
- Disposal: if the sparkler fails to prepare off the thermite mixture, dispose of the unreacted mixture past pouring it into dilute (ii Chiliad) hydrochloric (1200 cmthree) or sulfuric acrid (600 cm3) in a beaker in a fume cupboard and leave to stand overnight. Yous tin can apply 1 Grand dilute acrid but will demand to double the volumes of acid used. Filter off the solids and so place in the not-recycling waste and dilute downwardly the liquid with lots of h2o.
Procedure
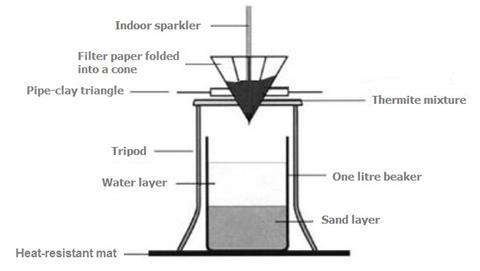
- Fold one 11 cm bore filter paper into a cone shape.
- Into a 1 dmthree beaker, pour sand until information technology is two-fifths (ii/v) full and so add water until it is 4-fifths (4/5) full.
- Comprehend an area of the demote with several heat-resistant mats and place the chalice in the centre. Fix upwardly the equipment as shown in the diagram above and surround it with condom screens. Add together the thermite mixture (see note 6) to the filter paper cone sitting in the piping clay triangle. Warning: push the filter paper cone into the triangle firmly, then at that place is no tendency for it to pop out.
- Insert a domestic sparkler upright into the thermite mixture. 3–4 cm should be buried beneath the mixture with the rest extended above the filter paper cone. Low-cal the sparkler with a Bunsen burner flame and retreat to a safe distance behind the condom screens. A very vigorous reaction should follow, with some sparks flying upwardly. The very hot balance containing molten iron volition fall through into the h2o.
- Once the reaction has stopped, remove the beaker and decant the water into the sink. Recollect the iron formed with a magnet. Wash the iron under running water.
Teaching notes
The reaction is: iron(3) oxide + aluminium → aluminium oxide + atomic number 26.
This shows that aluminium is to a higher place iron in the reactivity series.
In one case underway, the reaction is highly exothermic, rapidly reaching temperatures as high every bit 2000 °C, well in excess of the melting bespeak of atomic number 26 (1535 °C).
The practical use of this reaction is to weld railway lines together and could be mentioned.
The 'thermite' mixture is stable until strong heating is applied, hence the need for the domestic sparkler to initiate the reaction. Some of the igniter mixtures normally used in the by are now covered by the United kingdom of great britain and northern ireland Explosives Regulations 2014. This means that no more 0.5 g of such mixtures can exist used unless the school has an Explosives Certificate issued past the police. The sparkler method is preferred because igniter mixtures on calibration of 0.5g or below are less reliable.
If you lot choose to show students video clips of thermite reactions from the internet, take care to avoid those that are carried out on a scale and in a style which is hazardous.
Boosted data
This is a resource from the Practical Chemistry project, adult past the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including total technical notes and step-by-step procedures. Practical Chemistry activities accompany Applied Physics and Practical Biological science.
The experiment is too role of the Royal Society of Chemistry's Standing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry
Health and safe checked, 2016
This resource has been reviewed and updated with advice from CLEAPSS in response to the United kingdom Explosives Regulations 2014.
Iron Oxide Reacts With Aluminium,
Source: https://edu.rsc.org/experiments/the-thermite-reaction-between-aluminium-and-ironiii-oxide/724.article
Posted by: aginpegare.blogspot.com


0 Response to "Iron Oxide Reacts With Aluminium"
Post a Comment